JDRF Canada, in collaboration with CIHR, is thrilled to announce the recipients of the CIHR-JDRF Type 1 Diabetes Screening Research Consortium. This $12 million grant will develop a single nationally coordinated research network to explore key research questions about the feasibility and acceptability of general population screening for early-stage T1D in Canada. The consortium will build on experiences from other countries with T1D screening programs including the US, UK, Israel, Australia, and multiple European countries.
Most T1D screening studies have focused only on family members, who are at higher risk of T1D than the general population. However, as 90% of people diagnosed with T1D do not have any family history, family-based screening does not identify most people in the population who go on to develop T1D. This new funding opportunity looks to address this gap and will help to determine approaches for identification of Canadians with early-stage T1D who could benefit from education, monitoring and – in the future – therapies that could delay or even prevent the need for insulin therapy. As well, it will help advance research into potential disease-modifying therapies that could be applied when an individual is identified as high risk and could delay or prevent the need for insulin therapy.
JDRF is pleased to announce that Dr. Diane Wherrett (Toronto, ON) will lead CanScreenT1D – the Canada-wide T1D Screening Research Consortium team.

Dr. Wherrett is a physician in the Division of Endocrinology, Department of Paediatrics at The Hospital for Sick Children (SickKids) and a professor at the University of Toronto. She is the Canadian Centre Director for T1D TrialNet (an international research network that screens relatives of people with T1D and leads clinical trials of preventative therapies). The new Canadian consortium is made up of over 30 members including academic and clinician researchers, endocrinologists, people with lived experience of T1D, and knowledge users including a diabetes nurse, genetics counsellor, and a Ministry of Health representative. The acceptability of T1D screening in Indigenous communities will be explored, as led by Sasha Delorme of Diabetes Action Canada’s Indigenous Patient Circle and Indigenous people with lived experience of diabetes.
CanScreenT1D team leads are:
- Dr. Pranesh Chakraborty, Children’s Hospital of Eastern Ontario (CHEO)
- Dr. Robin Hayeems, SickKids
- Dr. Monika Kastner, University of Toronto
- Dr. Audrey L’Espérance, École Nationale d’administration publique
- Dr. Despoina Manousaki, Hôpital Sainte Justine
- Dr. Ashish Marwaha, Alberta Children’s Hospital Research Institute
- Dr. Jon McGavock, Children’s Hospital Research Institute of Manitoba
- Dr. Peter Senior, University of Alberta
- Dr. Albert Tsui, University of Alberta
- Dr. Bruce Verchere, BC Children’s Hospital Research Institute
- Dr. Holly Witteman, Université Laval
- Conrad Pow, North York General Hospital, Diabetes Action Canada
- Sasha Delorme, Diabetes Action Canada
CanScreenT1D will study different screening approaches, as well as the effectiveness of education and follow-up of people with early-stage T1D. CanScreenT1D will explore how general population screening for early-stage T1D could be carried out in Canadian health care systems, and conduct pilot studies of approaches to inform future implementation across Canada.
JDRF will work closely with the Screening Research Consortium to ensure that any opportunities for public participation in research consultation or patient engagement are distributed to our community. The pilot screening program is estimated to start in Fall of 2024.
The Importance of Screening
General population screening offers the potential to identify people who have early-stage, pre-symptomatic T1D. Canada has one of the fastest growing rates of T1D diagnoses anywhere in the world – and we don’t know why.
“Thanks to our team of researchers and patient partners across Canada, we are creating a pilot screening program to help identify children at risk of type 1 diabetes, aligned with the values and preferences of Canadians. With earlier diagnosis and connections with ongoing research initiatives, we can hopefully prevent serious complications at the time of diagnosis and increase access to treatments that may delay or prevent type 1 diabetes” – Dr. Wherrett
JDRF-funded research previously discovered that the presence of two or more specific markers indicative of an autoimmune response to the pancreas – called autoantibodies – indicates that a person is almost 100% likely to develop T1D in their lifetime. Screening provides the opportunity to educate those with early-stage disease about the signs and symptoms of T1D and provide supportive follow-up, preventing the life-threatening complication diabetic ketoacidosis (DKA) at diagnosis. With the FDA approval of Tzield, the first ever disease-modifying therapy for T1D, for people with early-stage disease, screening offers the opportunity to delay the onset of T1D diagnosis and further research into more disease-modifying therapies.
The prevailing medical wisdom used to be that T1D developed quickly, with a sudden onset of symptoms including thirst, hunger, increased urination, weight loss, and fatigue. Thanks to advances in screening and a better understanding of the human immune system, we now know that T1D does not develop suddenly but in fact the disease process usually starts long before insulin is required.
Once the immune system begins to attack the insulin-producing cells in the pancreas, we can detect markers in the blood (autoantibodies) that tell us a person is at increased risk. This is because the disease is otherwise asymptomatic or silent earlier on.
T1D happens in 3 stages:
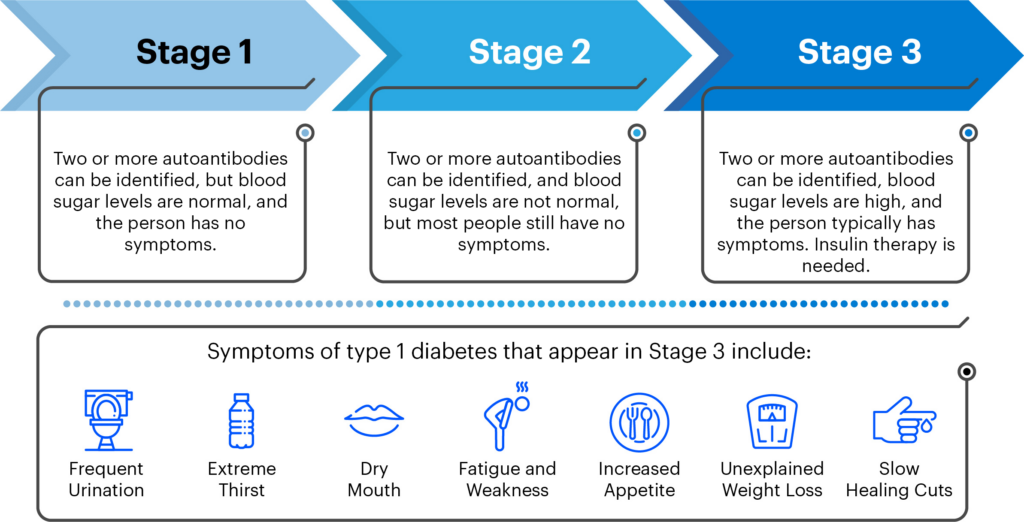
Because most people do not have a family history of T1D, symptoms and a diagnosis often come out of the blue. In 25-45% of diagnoses in children in Canada, this unexpected diagnosis comes with DKA, a serious and life-threatening complication that can lead to death if not treated promptly. An important part of a screening program will be follow-up monitoring for those who screen positive for T1D autoantibodies, to lower the risk for life-threatening DKA at diagnosis and serious complications, and accelerate the evaluation of disease-modifying therapies that could delay or prevent the disease.
A key goal of JDRF’s global research strategy is to support research that enables introduction of general population screening to identify high-risk individuals for early detection, reduce DKA at diagnosis, and accelerate the evaluation of disease-modifying therapies that could delay or prevent the disease.
JDRF has numerous research studies examining the efficacy of potential disease-modifying therapies for T1D. But many of these therapies will work best during stage 1 and 2 T1D, which can only be identified via a screening program. Stopping T1D before it starts is the ultimate goal, and a universal screening program will be essential to prevent new diagnoses of this disease in the future.
Current Screening Options in Canada
Currently, only family members of people with T1D can be screened for T1D risk through the TrialNet research program. TrialNet is an international network of leaders in T1D research and clinical care with centers in the United States and internationally.
We strongly encourage you to consult with your or your child’s physician for input as you make decisions about screening for T1D risk. Considering various sources of expert guidance and that from one’s own physician is the best way to make personal health choices.