Project ACT
Project ACT (Accelerate Cell Therapies) is Breakthrough T1D’s global plan to make curative cell therapies a reality for people living with type 1 diabetes (T1D).
What is Project ACT?
Project ACT is a coordinated, worldwide effort to dramatically speed the development, approval, and adoption of cell therapy treatments for T1D. Our work spans research and development, clinical studies, regulatory approval, access, and readiness for implementation.
Cell therapies have the potential to cure T1D. They involve replacing the glucose-sensing, insulin-producing cells that are destroyed in people with T1D. By investing in science and aligning efforts across our global network, Breakthrough T1D is accelerating the path to these transformative therapies.
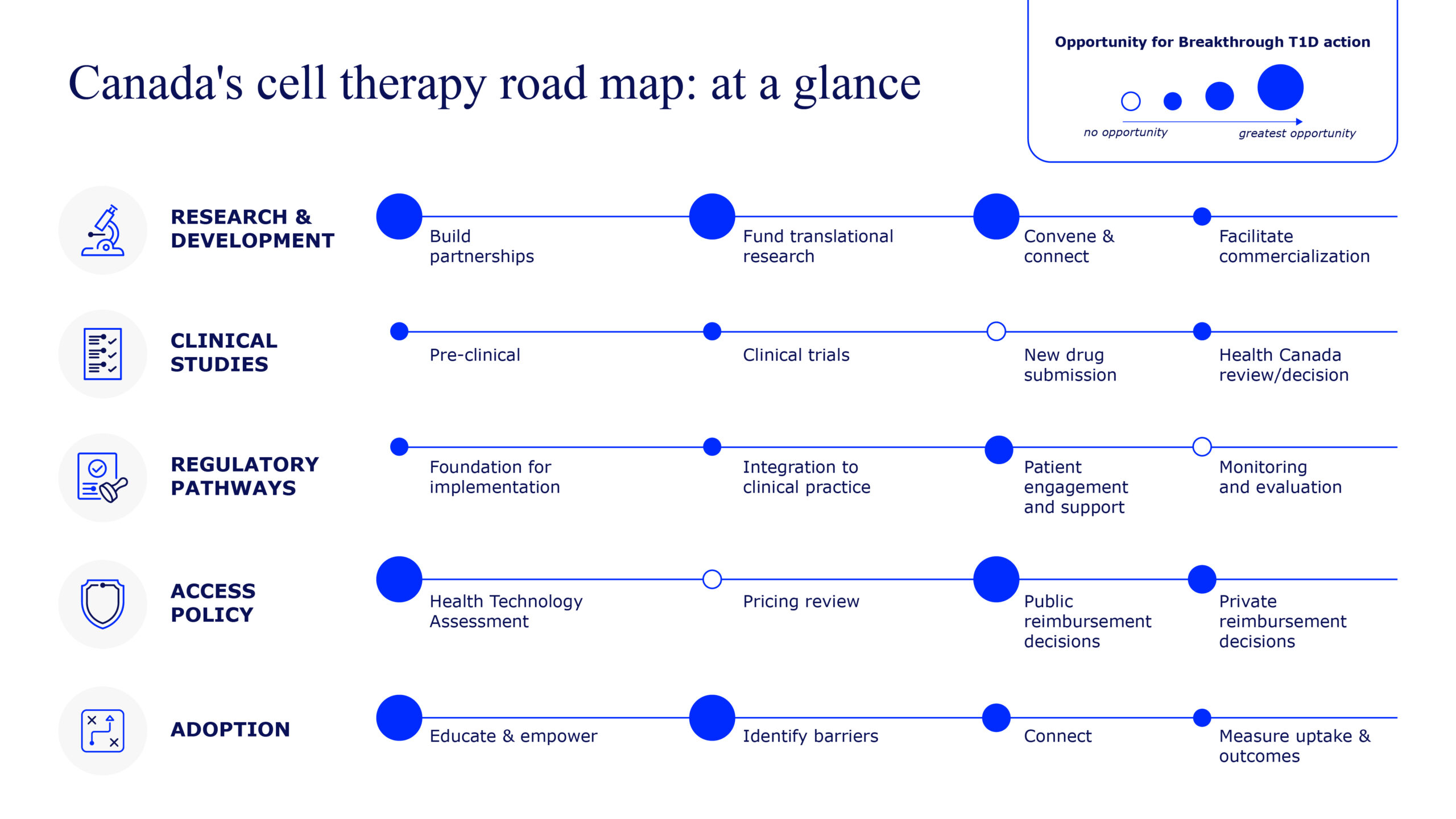
Canada’s Leadership
Canada has played a defining role in diabetes research—from discovering insulin, to pioneering stem cell science, to developing the Edmonton Protocol for islet transplantation and treating hundreds of people with T1D across the country. With this unmatched legacy and deep expertise, Canada is central to advancing cell therapies globally.
But developing effective therapies is only one piece of the puzzle.
Canada must also be ready to evaluate, approve, and deliver these treatments equitably. That’s why Breakthrough T1D is not only funding research but also preparing health systems for access and adoption.
Breakthrough T1D Canada’s Cell Therapy Roadmap will direct and focus the objectives of Project ACT in Canada.
Read more about how we’re working to reduce hurdles to cell therapies in Canada.
We fund cutting-edge research focused on producing healthy insulin-producing cells at scale, protecting them from the immune system, and ensuring they survive long-term after transplantation. Breakthrough T1D support helps researchers move promising discoveries toward clinical testing and encourages sharing of knowledge to speed progress.
Canada is home to world-leading cell therapy researchers, including those at the Breakthrough T1D Canucks for Kids Fund Centre of Excellence at the University of British Columbia.
Breakthrough T1D advances clinical research by funding studies, partnering with other organizations, and engaging people with T1D to ensure trials measure outcomes that matter most. We also help connect people who want to participate in research.
With its long history of islet transplantation and a strong research community, Canada is an excellent place to test stem cell–based therapies.
Traditional regulatory processes were not designed for cell therapies, which behave differently from drugs or medical devices. Through Project ACT, we are partnering with Health Canada and other decision-makers to build regulatory pathways that support timely evaluation and approval of cell therapies.
We will also work with the T1D community to ensure their priorities and lived experiences inform regulatory decisions.
To make cell therapies available, governments and insurers must determine who qualifies and how therapies will be funded. Because these treatments are complex and novel, they may require new approaches to access and reimbursement.
Breakthrough T1D is committed to partnering with decision-makers to ensure equitable access. We will work with people living with T1D to demonstrate the full value of cell therapies and build a strong case for public and private coverage
Most health systems are not yet equipped to deliver T1D cell therapies, which require specialized skills and coordinated care. Through Project ACT, we will:
- Train health care professionals to ensure clinical readiness
- Support rapid adoption once therapies are approved
- Help establish strategically located clinical centres to deliver these treatments safely and effectively
We are also committed to supporting informed decision-making within the T1D community. Because cell therapies are not yet widely understood, we will partner with people with T1D and caregivers to understand their questions and concerns, and to provide clear, unbiased, evidence-based information.
Why Breakthrough T1D?
Because revolutionizing T1D treatment is what we’ve always done.
For more than 50 years, Breakthrough T1D Canada and its affiliates have contributed to nearly every major advancement in T1D research. These breakthroughs have added roughly 25 years to the life expectancy of many Canadians with T1D—but cures are still needed.
We will help deliver cures through cell therapies.
Curing T1D has been our mission since the beginning. We were founded by families determined to create a better future for their children, and we will not stop until a world without T1D becomes a reality.
Many of the cell therapies now in human clinical trials—including those developed by Vertex, were made possible by Breakthrough T1D and our T1D Fund – A Breakthrough T1D Venture.
Together with our donors, academic partners, governments, other funders, and the strength of the T1D community, Project ACT will bring transformative treatments to people living with T1D.