We are pleased to announce 6 new grants funded via Breakthrough T1D International (USA) to stellar Canadian scientists. These grants all support cell therapy research to replace insulin-producing cells in people living with T1D.
Dr. Cristina Nostro, University Health Network (Toronto)
Manufacturing clinical-grade stem cell-derived islets – 2 Grants
To date, global efforts on this work – largely led by Canadians – has used research-grade cell lines which are less expensive and more readily available for experimentation. To move these therapies towards the clinic there is a requirement for Good Manufacturing Practices (GMP) to be met. This includes properly trained personnel, GMP-certified premises and equipment, clinical-grade starting cell lines, validated protocols and practices, etc.
Two shots on goal: These two grants will each use Dr. Nostro’s world-renowned differentiation protocol to engineer islets using clinical-grade stem cell lines in GMP-certified facilities that have the ability to scale up the differentiation process.
Consider Dr. Nostro’s differentiation protocol as an excellent sourdough bread recipe perfected in her kitchen with consistent, delicious results. That recipe will now be taken to two different commercial bakeries to use their ingredients and facilities to see if they can make the same excellent sourdough loaves consistently in large production quantities.
Dr. Guy Rutter, CHUM Montreal
Imaging to explore the survival and function of stem cell-derived islets transplanted in mice
Goal: A novel imaging technique to examine human stem cell-derived islets in mice
- Will allow real-time comparison of transplant sites (liver vs under skin)
- Better assessment of cell death / blood supply / immune attack
A significant hurdle for assessing T1D and islet transplantation is that there is virtually no way to ‘see’ islets inside a person, even in small animals. No imaging technique allows us to ‘look at islets’ and see how many are alive, functional, producing insulin, etc. This is why some clinical trials using islet transplantation devices (microencapsulation) have involved device removal at periodic timepoints so that the islets can be removed from the body and examined to evaluate their survival and function. Dr. Rutter is proposing a novel imaging technique to ‘see’ islet functioning in mice, which can help guide factors affecting the survival and function of transplanted islets.
Dr. Patrick MacDonald, University of Alberta
Artificial Intelligence (AI)-driven benchmarking for understanding and improving stem cell-derived islets
Over the past 5+ years, Dr. MacDonald and his team have created a groundbreaking database: humanislets.com, which isolates islets from donated pancreases (from deceased donors with T1D, T2D, and without diabetes) and characterizes the islets and their function using “omics”. This is a broad term to encompass the study of all the biological molecules of a cell, such as genomics (DNA), transcriptomics (RNA), proteomics (proteins), and metabolomics (metabolites), etc. Together “omics” data creates an exceptionally robust characterization of each islet – and all of this is made publicly available to scientists around the world through this research.
Goal: Dr. MacDonald’s new grant will expand this Breakthrough T1D-funded database to include stem cell-derived islets for comparison to donor islets and incorporate artificial intelligence methods to dig deeper into this comparison.
Dr. Haoning Cen, University of British Columbia
Postdoctoral Fellowship (Supervisors: Dr. Francis Lynn & Dr. Leonard Foster)
A blueprint for making better beta cells
Goal: to create blueprints for donor beta cells vs stem cell-derived beta cells
- Will use protein detection to identify 10,000+ proteins
- Will classify each protein’s quantity and location to compare ‘natural’ vs stem cell-derived beta cells
Dr. Sean Kinney, University of Toronto
 Postdoctoral Fellowship (Supervisor: Dr. Michael Sefton)
Postdoctoral Fellowship (Supervisor: Dr. Michael Sefton)
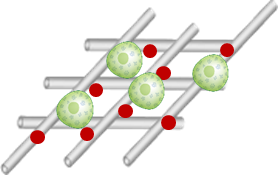
Biomaterial platform for islet transplantation under the skin
Goal: to create a biological scaffold (a small structure made from bio-compatible materials that can support new cell growth and survival) for islet transplantation using regenerative hydrogel.
- The scaffold will support blood vessel, nerve, tissue growth to support islet survival
- Immunomodulating therapy (modifying the immune system’s function, for example) embedded in the scaffold will be explored