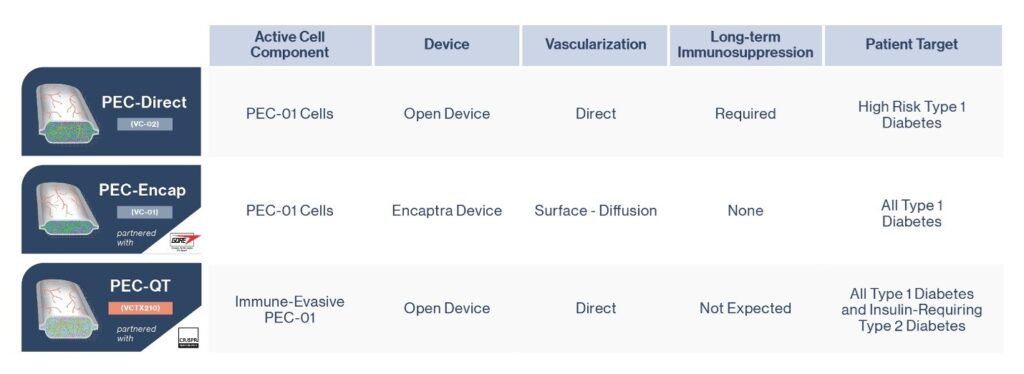
*Image taken from viacyte.com/pipeline/
A significant focus of JDRF is funding the most promising research that could lead to potential cure therapies for type 1 diabetes (T1D).
ViaCyte, a cell replacement company long supported by JDRF, has three separate stem cell replacement therapy products in development, all with the aim of reducing or eliminating the need for external insulin for people with T1D.
Researchers look for ways to use stem cells as a renewable source of insulin-producing cells which, when transplanted, would replace the beta cells that are destroyed in a person with T1D, allowing them to produce insulin again. This would lessen or eliminate the amount of external insulin required by someone living with T1D (either by injection, pen, or pump) for months or even decades.
The biggest challenges to stem cell replacement therapy are identifying the appropriate stem cell source (i.e., pancreatic cells, or liver cells) and ensuring that they both function well and will not be rejected by the recipient’s immune system. Much like a transplanted organ – most stem cell replacement therapies require immunosuppressing medications to prevent rejection.
Updates on ViaCyte Clinical Trials
PEC-Encap (VC-01™; see middle figure) was ViaCyte’s first product including a stem-cell derived precursor that once implanted in humans has demonstrated that they will mature into beta cells that produce insulin.

The company’s second technology, PEC Direct (VC-02™; see top figure), has now been the subject of two papers that report the preliminary results of its clinical study, which involved an international team of clinicians and researchers including several at the University of Alberta and at the University of British Columbia’s Faculty of Medicine and Vancouver Coastal Health (VCH).
The UBC-VCH study is part of larger international clinical trials led by ViaCyte, which is also studying the effectiveness of cell-replacement therapy on participants in sites across Canada, the U.S. and Belgium.
One of the new papers reports on 15 trial participants studied at the Vancouver site. At the start of the UBC-VCH study, each participant had several ViaCyte cell-containing devices implanted just below the skin. Each device, thin as a credit card, contained millions of lab-grown cells that originally came from a single stem cell line. These cells had been trained to mature into insulin-producing beta cells.
Six months after implantation, the cells had not only survived but successfully matured into insulin-producing beta cells, which helped the trial participants to sense blood sugar levels and release insulin when needed.
This study used levels of C-peptide (released into the blood as a byproduct of insulin production) to measure insulin produced by the implanted cells. The researchers found C-peptide levels rose after patients ate a meal—evidence of normal beta cell function. This is significant as external insulin (either received via injection, pump or pen) does not generate C-peptide.
Participants also spent 13 per cent more time in target blood sugar range and some were able to reduce the amount of insulin they injected.
The insulin produced through the implanted cells was not enough for participants to forgo external insulin altogether, but the cells did survive and maintain function a full year post-transplantation. This demonstrates the potential for durability of this kind of cell replacement therapy, and may ideally prevent frequent implantation, which must be done surgically. Importantly, the trial did not reveal any safety concerns.
This research was supported by funding from JDRF Canada, as well as Canada’s Stem Cell Network, Vancouver Coastal Health Research Institute, Canadian Institutes of Health Research, ViaCyte Inc. and California Institute for Regenerative Medicine.
What does this mean for people with T1D?
These studies offer hope and cautious optimism about cell replacement therapy as a possible pathway to a T1D cure.
“Our findings demonstrate the incredible potential of this stem cell-based treatment. With further research, this treatment could one day eliminate dependence on insulin injections and transform the management of Type 1 diabetes,” said the study’s senior author Dr. Timothy Kieffer, professor in UBC faculty of medicine’s departments of surgery and cellular and physiological sciences, who was recently appointed as ViaCyte’s chief scientific officer.
The next step is researchers need to determine what cells are optimal for transplantation, and the best transplantation site. There also needs to be further study on how long the cells work effectively, remain safe, whether ta greater number of cells is required for long-term insulin production, and whether it is possible to eliminate immunosuppressive therapy.
A third ViaCyte cell replacement product including a gene-edited stem cell source, VCTX210, developed in partnership with CRISPR Technologies, was recently approved for clinical trial by Health Canada. Unlike the PEC-Direct product used in the newly published results, this product would not require immunosuppression – a key next step on the cell replacement roadmap. (See Figure 3)
Read more here: breakthrought1d.ca/exciting-news-about-new-health-canada-approved-clinical-trials/
The ViaCyte clinical trials are one of several potential cell replacement cures therapies JDRF supports globally, as part of its overall research strategy. Read more about it here: breakthrought1d.ca/research/



