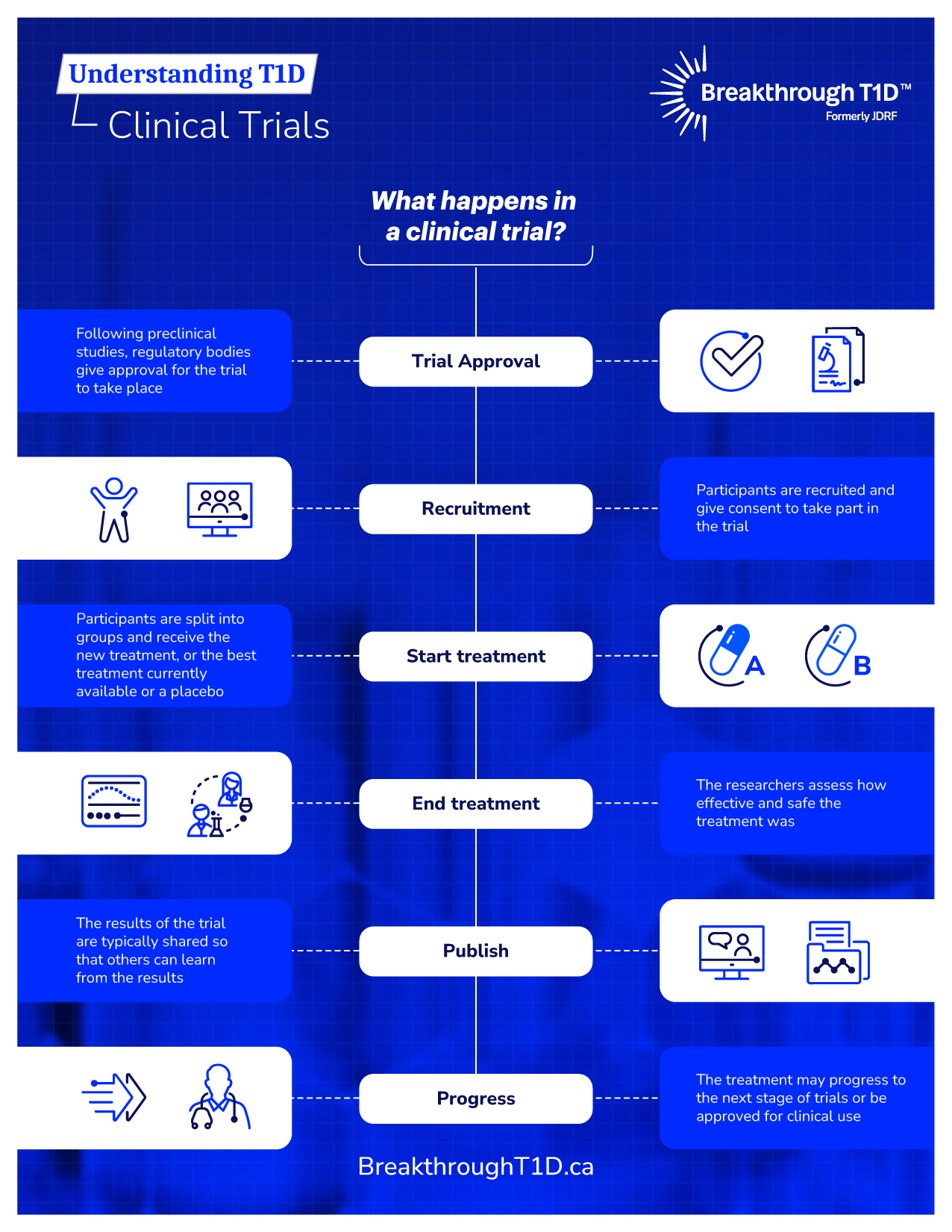
Clinical trials
A key component of Breakthrough T1D’s research strategy is the support of clinical trials because they help advance work that has been tested extensively in the lab to testing in people. These real-life studies are when we truly put new therapies designed to prevent, treat or cure type 1 diabetes (T1D) to the test.

How can I contribute to clinical research?
One of the biggest challenges for clinical trials is finding volunteers to take part in studies. 80% of T1D trials are delayed, largely because of lack of participants. Choosing to participate in a clinical trial is a very personal decision. Early access to promising new treatment can be an enormous benefit. Others have found that by participating in a clinical trial, they learned more about their health or T1D management. By volunteering to participate in a clinical trial, you are helping all people living with T1D, by enabling research towards better health outcomes and cures.
Find a T1D clinical trial
Learn more about actively recruiting T1D clinical trials in Canada.
Your questions about clinical trial participation answered
Despite what many believe, some clinical trials do not use ‘inactive’ placebos, like a sugar pill; some compare a potential new therapy against an already approved therapy. And participants who do receive a placebo still receive personalized, quality care from top doctors as part of the study and may be offered the intervention after the study ends if it’s found to be effective.
Concern that participation in a clinical trial could affect diabetes management can be a barrier to clinical trial recruitment.
Clinical trials may provide an opportunity to access brand new medical advances. In some cases, participants can continue the therapy after the end of the trial if it’s found to be effective. Most participants experience a benefit in diabetes management or understanding their condition just from being enrolled in a trial since they often receive frequent, high-quality care at top facilities throughout the duration of the trial.
Interventional trials are actively ‘giving’ something to the participants (such as a drug, a surgery, an exercise program, access to a mental health app, etc.). These studies are looking for the effect of the intervention. Observational trials are just ‘recording’ something from the participant (such as taking blood for screening, asking for demographic information in a registry, etc.).
While an interventional trial is more ‘active’, versus a more ‘passive’ participation in observational trials, both provide critical information that helps to advance understanding of T1D and improve treatment outcomes.
Side effects are possible for all clinical trials – this is one of the reasons that these trials are conducted. Any anticipated side effects are detailed prior to beginning the study in the informed consent document. Any side effects that occur are recorded and managed very closely, often by a dedicated safety committee.
All clinical trials go through rigorous ethical assessment and, when required, Health Canada approval. Before joining a clinical trial, you will be asked to sign an informed consent form, which outlines the details of the trial, but this is not a binding contract—even after you sign it, you can leave the trial at any time, for any reason.
Concerns about equity can be a real barrier to clinical trial participation.
Clinical trials and the resulting interventions or observations are safer and more effective for everyone when diverse populations are included. All clinical research is HIGHLY scrutinized for ethical treatment of participants and participants will receive equal care regardless of age, sex, gender, sexual orientation, ethnicity, race, diabetes management practices, etc. Study teams keep the treatment of participants as EQUITABLE as possible to not influence the results.
You may think that living in a rural or remote area prevents you from participating in clinical trials, but that is not the case. Many clinical trials have travel budgets to cover participant expenses such as driving/flying costs, accommodation, parking, sometimes even food and time compensation. There are also several clinical trials that can be done from the comfort of your home through virtual platforms.
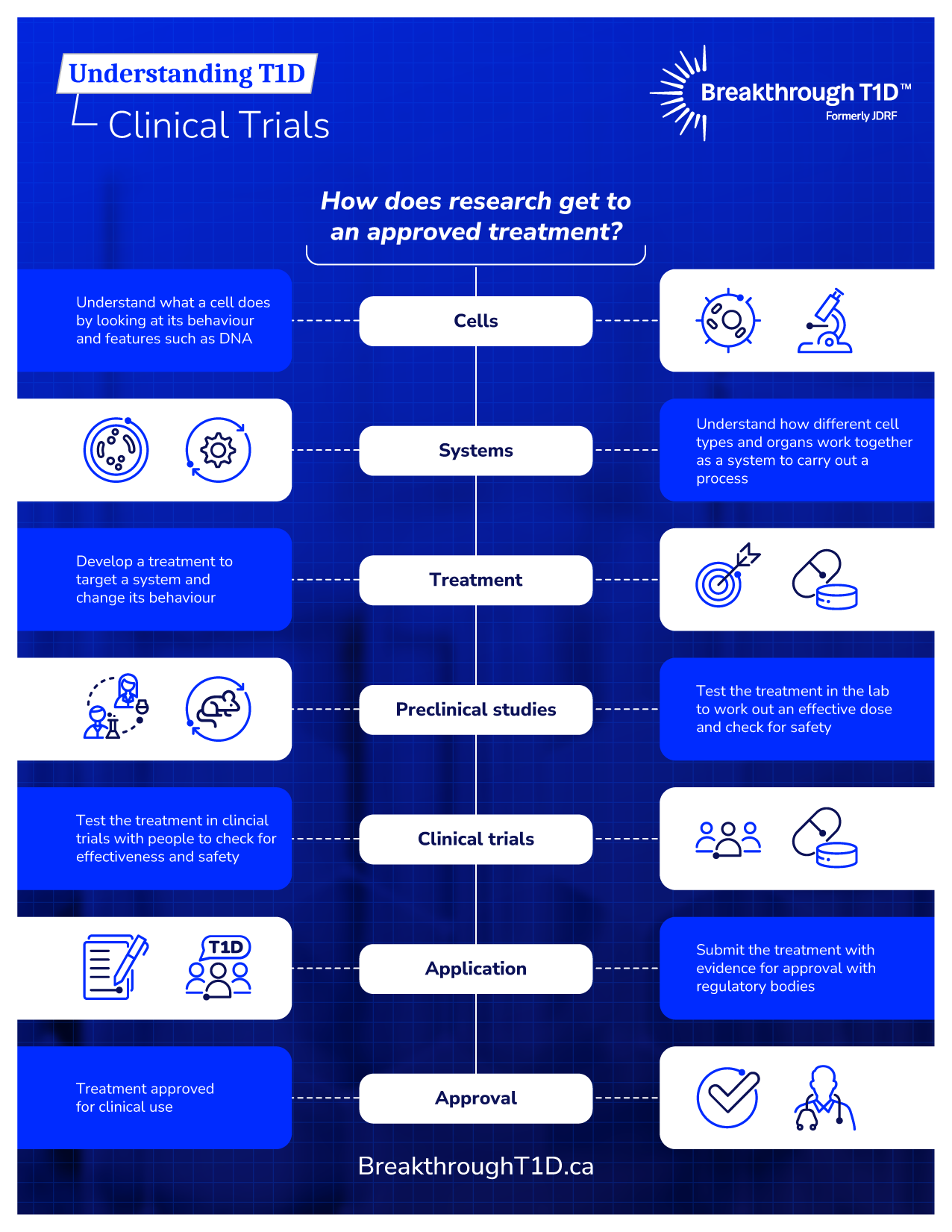
Legacy of Breakthrough T1D-Funded Trials in Canada
Canadian clinical researchers have made an immeasurable impact upon the lives of those with diabetes, ever since Dr. Banting and Best first discovered the insulin hormone and its role in diabetes. A highlight in our legacy is Breakthrough T1D Canadian Clinical Trial Network (formerly JDRF CCTN), which was established in 2009 to accelerate innovative solutions for the management, care, and cure of T1D.
Created in partnership with the Government of Canada, funding for the network came from a commitment of $20 million by the Federal Economic Development Agency for Southern Ontario (FedDev Ontario), with an additional $14 million contribution from Breakthrough T1D (then known as JDRF). The $34 million investment is accelerating the testing of new technologies and treatments for Canadians and individuals around the world living with T1D and its complications. A generous $3 million investment from the WB Family Foundation later enabled the network to expand into western Canada, helping to fund new trials in Alberta and BC.
Breakthrough T1D Canadian Clinical Trial Network has supported over a dozen clinical trials, as well as multiple training awards and diabetes device projects. Clinical trials enabled by this project include CONCEPTT, which has led to use and coverage of continuous glucose monitors in pregnant women with T1D in many countries; ViaCyte’s first trial of encapsulated, stem cell-derived therapies; and trials testing whether a drug called ustekinumab can slow disease progression in young adults just diagnosed with T1D (in progress).