Adolescence can be a challenging time to manage type 1 diabetes (T1D). Life (and hormones!) change in all sorts of ways, and many teenagers experience higher than recommended blood glucose levels as a result, which can mean an increased risk of complications later in life. The study of novel therapies that can improve glycemic control in teens with T1D and reduce the risk of diabetes complications and is critical to improving the lives of youth living with diabetes.
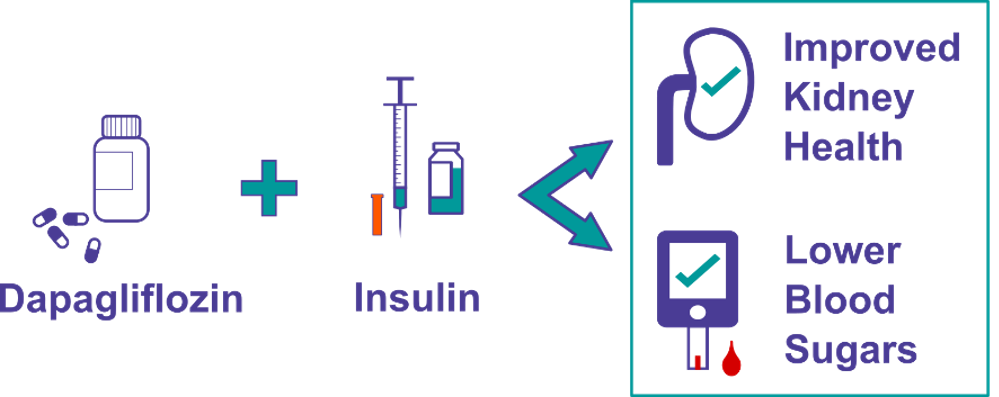
Adjunct-to-insulin therapy – ie, taking another drug alongside usual insulin treatment – is one approach that could help on both fronts. For example, SGLT2 inhibitors are a class of oral medications approved for type 2 diabetes that stop glucose from the blood from being absorbed by the kidneys, instead encouraging glucose to be released in urine. Dr. Farid Mahmud and his team at the Hospital for Sick Children in Toronto are now recruiting for a JDRF-funded clinical trial that will test the safety and efficacy of an SGLT2 inhibitor called dapagliflozin in teens with T1D.
The research team is seeking participants aged 12-18 years with established type 1 diabetes in the Greater Toronto Area (Hospital for Sick Children) and Southwestern Ontario (Children’s Hospital of Western Ontario, London) who may be eligible to participate in the Adolescent Type 1 Diabetes Treatment with SGLT2i for Hyperglycemia & Hyperfiltration trial – also known as ATTEMPT attempt.study@sickkids.ca.
SGLT2 inhibitors such as dapagliflozin can improve blood sugars, increase time in range and decrease kidney pressure, called hyperfiltration. Studies in adults with T1D have shown that SGLT2 inhibitors can lower HbA1c, insulin dose and weight. By alleviating glucose absorption in the kidneys, these drugs can also help prevent long term damage to these organs.
What is the ATTEMPT study?
ATTEMPT is a 22-week clinical trial that aims to determine the safety and effectiveness an SGLT2 inhibitor called dapagliflozin on managing blood glucose and on improving kidney function in adolescents aged 12 to 18 with T1D. The study is being conducted to determine how this therapy can benefit and be used effectively in adolescents with T1D.
ATTEMPT is led by Dr. Farid Mahmud, an endocrinologist and researcher at The Hospital for Sick Children in Toronto. Dr. Mahmud’s overall research focus is diabetes, clinical and translational research relating to other autoimmune conditions (such as celiac disease), and early evaluation and prevention of diabetes-related complications. His research interests include the evaluation of medication and lifestyle interventions in high-risk pediatric groups and the evaluation of impact of the social determinants of health in youth with diabetes. He is also actively engaged in patient-centered research as part of the CIHR Strategies for Patient Oriented Research (SPOR- Can-SOLVE CKD) and as part of the JDRF-funded AdDIT (Adolescent Diabetes Cardio-renal Intervention Trial).
What does joining the ATTEMPT study entail?
Once enrolled in the study, a participant can expect:
- 5 in-person visits over 22 weeks
- A random assignment to the dapagliflozin group, or the placebo group (a small pill that contains no active medicine)
While part of the study, participants will:
- Keep taking insulin
- Wear a continuous glucose monitor (CGM)
- Test for blood ketones
- Report any adverse events
Study participants will be compensated and provided support for costs associated with travel or parking. To learn more about the study and how to enroll, please contact: 416-813-7654 ext. 204517 or email attempt.study@sickkids.ca