Since 1986, corporate Canada has been participating in one of Canada’s longest running and top fundraising events, the JDRF Ride to Defeat Diabetes. This year rides were held in Montréal and Toronto on October 10 and 16 and 17, respectively, along with ‘Ride Your Way’ events taking place across the country all October. T1D is […]
T1D Research Updates from the September 2024 EASD Scientific Conference
From September 9-13th, 2024, scientists, healthcare professionals, and the diabetes community from around the world gathered in Madrid, Spain for the European Association for the Study of Diabetes (EASD) annual conference. The event was attended by researchers, clinicians, pharmaceutical and device companies, regulators and leaders in the diabetes field from more than 120 countries, and […]
Breakthrough T1D and Integrated Nanotherapeutics Collaborate to Advance Development of an Immune Tolerizing Therapeutic to Treat Type 1 Diabetes
JDRF Canada is pleased to share that our US affiliate Breakthrough T1D (formerly JDRF, headquartered in the US) has awarded an Industry Drug and Development Grant to Integrated Nanotherapeutics (“INT”, Vancouver, BC). Founded by academic scientists, including Dr. Bruce Verchere – lead of the JDRF Centre of Excellence at UBC – INT is a biotechnology […]
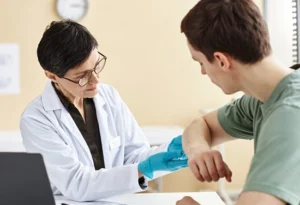
Psoriasis drug shows promise for treating type 1 diabetes
New research finds that ustekinumab, a drug commonly used to treat psoriasis, may help children and adolescents with type 1 diabetes keep making insulin for longer. Results from a clinical trial named USTEKID, published in the journal Nature Medicine, suggest that ustekinumab may be effective in treating the early stages of type 1 diabetes in children and […]
Faces of the $100M Campaign to Accelerate: Bonnie’s Story
Meet Bonnie Barber from Regina, SK, a resilient mother of seven, valued JDRF donor, and longstanding volunteer leader helping to champion JDRF Canada’s $100M Campaign to Accelerate. Bonnie shares her journey navigating the challenging early moments of her two children’s type 1 diabetes (T1D) diagnoses, how this relentless disease has impacted her family, and what […]
50-Day Challenge to Triple Donor Impact on Research
“Alone we can do so little; together we can do so much.” – Helen Keller, Disability rights advocate Since our founding 50 years ago, JDRF Canada has helped fuel almost every advancement in type 1 diabetes (T1D) care and cure research by bringing together the T1D community, donors, researchers, and funding partners to work towards […]
JDRF Canada Clinician Investigator Fellowship 2024 Recipient
The JDRF Canada Clinician Investigator Fellowship is open to medical residents that are pursuing an additional research degree (Master, PhD, or postdoctoral training) in the field of type 1 diabetes research. Residents must be part of their university’s Clinician Investigator Program (CIP) which is guided by The Royal College of Physicians and Surgeons of Canada. […]
Celebrating a 55th ‘Diaversary’ with an ambitious fundraiser
Miguel Alvarez, who has been living with type 1 diabetes for over 55 years, aims to complete two bike rides of over 100 miles each. To mark his 55th ‘Diaversary’ – the date when someone was diagnosed with type 1 diabetes (T1D), Vancouver, BC resident Miguel Alvarez will be attempting two 100-mile bike rides: the […]
Exciting updates from Vertex stem cell-based therapy clinical trials
On Friday, March 28, 2025 news from Vertex came announcing that they are discontinuing one of their T1D cell therapy trials. VX-264 is the trial of stem cell-derived islet cells that are transplanted in a macroencapsulation device (like tea leaves in a teabag) without the need for immunosuppression. Unfortunately, although the first phase of the trial was […]
Annual American Diabetes Association Conference provides updates on exciting developments in type 1 diabetes research
The American Diabetes Association’s 84th Scientific Sessions were held from June 21- 24th 2024. This annual conference brings together researchers and scientists to both present and learn about the latest in type 1 diabetes research and technological advancements. Many of the presentations features study results from researchers funded by Breakthrough T1D (formerly JDRF International) and JDRF […]
JDRF Walk to Cure Diabetes a huge success!
Throughout June nearly 50 communities gathered across Canada to raise funds for type 1 diabetes research. The JDRF Walk to Cure Diabetes is the largest fundraising event in Canada that brings together the type 1 diabetes (T1D) community to raise funds for T1D research. A fun-filled family and community celebration, the Walk has raised more […]
From Tragedy to Hope: Family Inspires New Mental Health Fund at Breakthrough T1D Canada
Content warning: This story is about a young man who tragically died from diabetes complications and experienced mental illnesses In May 2024, Evan Hunt laced up his running shoes and took the first of approximately 50,000 steps in the BMO Vancouver Marathon. He thought of his brother Brendan, who had undergone the pre-run ritual countless […]