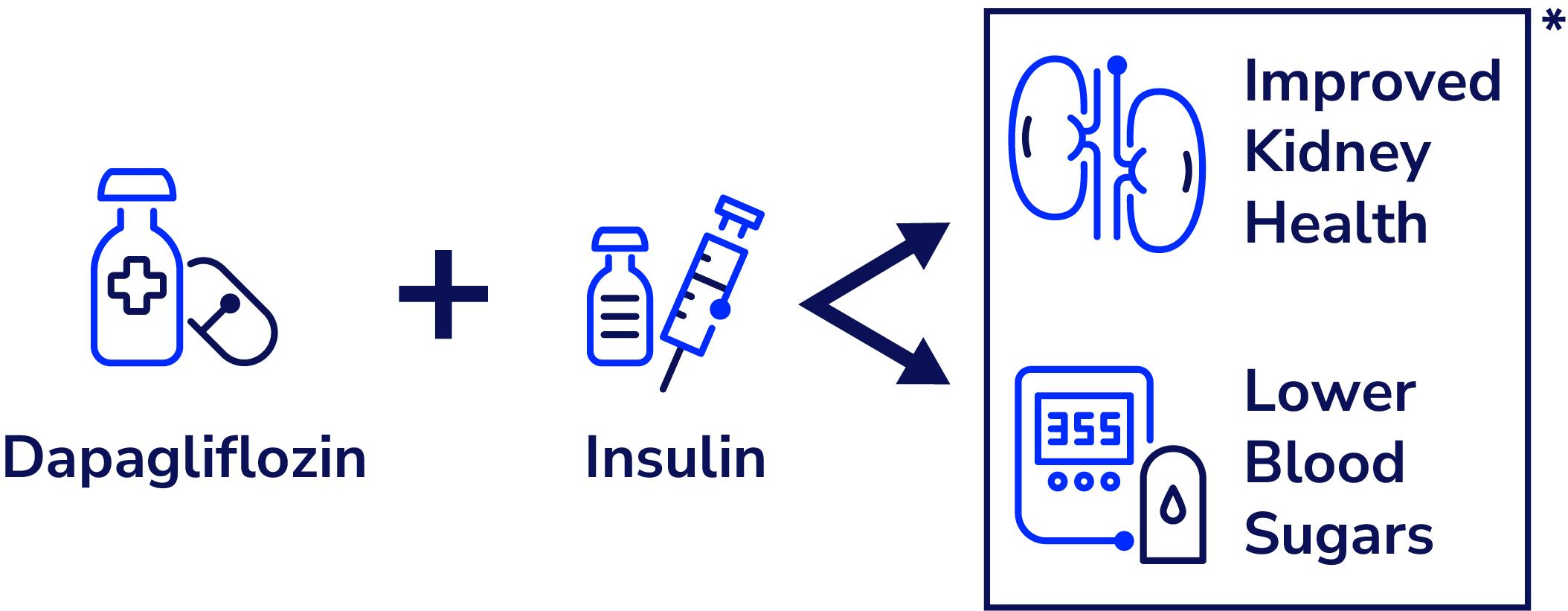
Dapagliflozin, an SGLT2i medication often used for people with type 2 diabetes, showed improved kidney function and glycemic management in teens with type 1 diabetes. This trial supports a growing body of evidence that supports the use of adjunctive therapies (drugs beyond insulin) for T1D.
Adolescence can be a challenging time to manage type 1 diabetes (T1D). Life (and hormones!) change in all sorts of ways, and many teenagers experience higher than recommended blood glucose levels as a result, which can mean an increased risk of complications later in life. The study of novel therapies that can improve glycemic control in teens with T1D and reduce the risk of diabetes complications is critical to improving the lives of youth living with diabetes.
Adjunct-to-insulin therapy – i.e., taking another drug alongside usual insulin treatment – is one approach that could help on both fronts. For example, sodium-glucose cotransporter-2 (SGLT2) inhibitors are a class of oral medications approved for type 2 diabetes that reduce glucose from the blood from being absorbed by the kidneys, instead encouraging glucose to be released in urine. Dr. Farid Mahmud and his team at the Hospital for Sick Children (SickKids) in Toronto conducted a clinical trial that tested the safety and efficacy of an SGLT2 inhibitor called dapagliflozin in teens with T1D. The ATTEMPT trial (Adolescent Type 1 Diabetes Treatment with SGLT2i for Hyperglycemia & Hyperfiltration) was funded as part of the Breakthrough T1D – CIHR Partnership to Defeat Diabetes.
The ATTEMPT study
The ATTEMPT trial aimed to determine the safety and effectiveness of an SGLT2 inhibitor called dapagliflozin on managing blood glucose and on improving kidney function in adolescents aged 12 to 18 with T1D. The study had two main goals:
- To determine how effective dapagliflozin would be at improving kidney function and glycemic management; and
- To determine if the drug increased the risk of diabetic ketoacidosis (DKA) and if additional safety measures could mitigate any increased risk
ATTEMPT was led by Dr. Farid Mahmud, an endocrinologist and researcher at The Hospital for Sick Children in Toronto.
A total of 98 participants and their families attended 5 in-person visits over 22 weeks and were given a random assignment to the dapagliflozin group, or the placebo group (a small pill that contains no active medicine). During the study, participants kept taking insulin, wore a continuous glucose monitor (CGM), tested for blood ketones, and were to report any adverse events. Over 850 potential participants were approached over the course of 2-years to take part in the study.
Results:
ATTEMPT is the first of its kind, landmark trial designed to evaluate the effectiveness of SGLT2 inhibitors to optimize diabetes control and prevent early subclinical kidney complications in an at-risk pediatric population with T1D.
Efficacy:
The study showed that a low dose of SGLT2 inhibitor could safely be given to youths and adolescents to improve kidney function as well as improve glycemic management. There was a clinically significant decline in HbA1c of 0.47% in the treatment group as well as a 9% increase in average time in range from CGM metrics. There was no change in the total daily insulin dose.
Safety:
The trial was designed with strict safety protocols to mitigate the risk of diabetic ketoacidosis. In collaboration with patient-partners (those living with and caregivers of individuals with T1D) a DKA Risk Mitigation Strategy was employed due to the increased risk of DKA during euglycemia (normal range glucose levels). The protocol included routine ketone monitoring with guidance for action above the threshold of 0.6 mmol/L.
Dapagliflozin was well tolerated with no study-related serious adverse events. There were no significant differences in the proportion of participants who experienced elevated ketone levels, hypoglycemia and genitourinary tract infections in the Dapagliflozin vs Placebo groups. A single case (N=1) of mild DKA was seen in the Dapagliflozin group. While rates of DKA were low, a greater number of elevated blood ketone events ≥0.6mmol/L were seen in the Dapagliflozin group (n=106) vs Placebo group (n=62) (P<0.001), demonstrating the importance of the patient-centered DKA Risk Mitigation Education strategy operationalized during the study.
The results from ATTEMPT will hopefully pave the way for further research and longer studies on the potential benefits of using adjunctive therapies to help manage type 1 diabetes.
Please note that dapagliflozin, or any SGLT2 inhibitors are not approved for individuals with T1D by Health Canada.
Mahmud, F.H., Bjornstad, P., Clarson, C. et al. Adjunct-to-insulin therapy using SGLT2 inhibitors in youth with type 1 diabetes: a randomized controlled trial. Nat Med (2025). https://doi.org/10.1038/s41591-025-03723-6
Clinical trial participation is crucial for moving forward vital T1D research from the lab to the people who need it most. To find T1D trials that are recruiting participants, and if you qualify, please visit: clinicaltrials.breakthroughT1D.ca.