
On March 27, 2025 Diabetes Canada released updated Clinical Practice Guidelines for the glycemic management across the lifespan for people with type 1 diabetes.
What are clinical practice guidelines and what do they mean for people with type 1 diabetes (T1D)?
Clinical guidelines are the guiding principles for health care practitioners to stay up to date on best practices in the management and care of people living with all diseases, including type 1 diabetes. As research advances, both in the medication and device landscape, clinicians and experts in the diabetes field meet to discuss the best available evidence and to craft or update the guidelines so that care is optimized.
Knowing your diabetes goals in the context of your life and your circumstances is the first step in deciding upon treatment options with your care team. As you manage your T1D, or the T1D of your family member, it is important to remember that your glycemic targets should be designed to fit into your life, and that conversations with your healthcare provider will help you understand the risks, benefits and considerations of all available treatment options.
This month’s updated guidelines, the first comprehensive update since 2018, are noteworthy because not only do they focus on managing one’s glucose levels across the lifespan, recognizing that children, teens and adults can all have T1D, they also include two exciting and important updates.
What are the new updates and what is their impact on Canadians living with T1D?
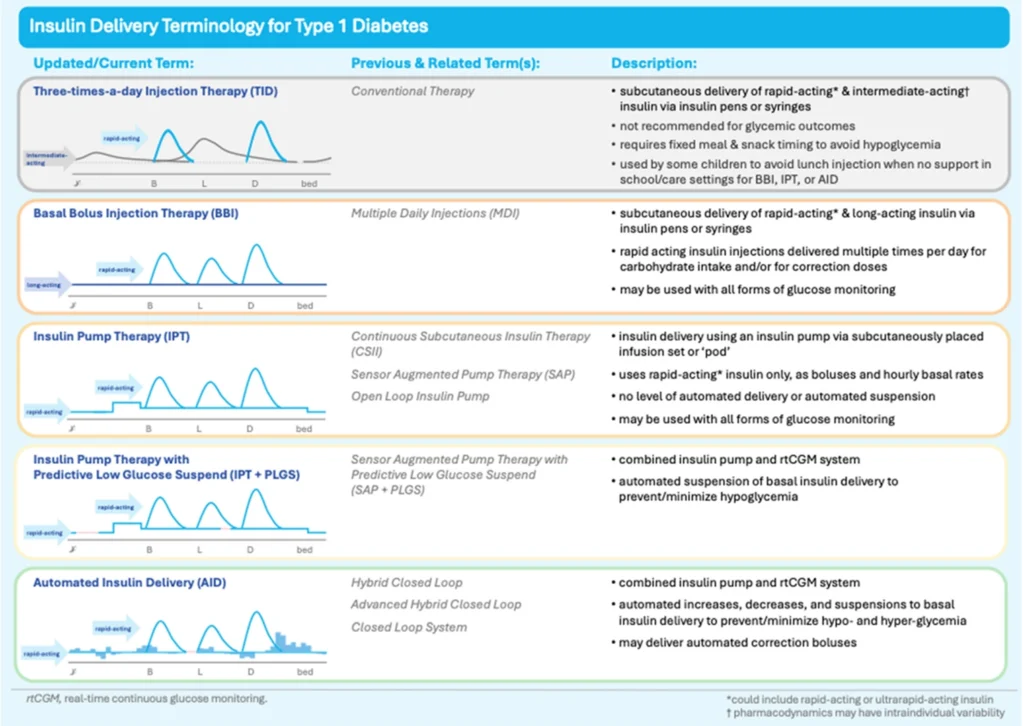
An AID system is a device that automatically adjusts insulin delivery based on blood glucose levels, measured from an accompanying paired glucose monitoring device. It’s also been called an artificial pancreas or hybrid closed-loop system, but AID is the preferred clinical terminology,
The updated guidelines now recommend that insulin be delivered ideally by an insulin pump integrated with a continuous glucose monitor (CGM) (AID) and that these devices be offered to all individuals with T1D, provided they are willing to wear the device and operate it:
Automated insulin delivery (AID) systems (insulin pump and connected continuous glucose monitor) are the preferred treatment method for all individuals to optimize glycemia and/or person-related outcomes, provided the individual is willing and able to wear and operate the devices.
The paradigm shift here is important, as previously the belief was that only those individuals who have met their glucose targets would benefit from this advanced technology. AID systems are beneficial for diverse individuals, regardless of their baseline, or starting glucose levels, and their age or socio-economic status for example. In other words, we need to change the thinking that implies a person with T1D needs to ‘earn’ an AID through already relatively stable management.
AIDs have been shown to keep glucose levels stable and in better range, while also improving sleep and reducing the burden of daily diabetes management (from reducing the fear of hypoglycemia, diabetes distress and improving overall quality of life).
Adjunct therapies for people with T1D
The second noteworthy recommendation is the first of its kind worldwide – adjunctive therapies may now be considered in adults with T1D, based on shared decision-making with the care provider. There is substantial research that has demonstrated the safety and effectiveness of these non-insulin agents such as metformin, glucagon-like peptide-1 receptor agonists (GLP1-RA) or sodium-glucose cotransporter-2 inhibitors (SGLT2i), or what you may know as Ozempic, Wegovy and other GLP-1 agonists, in type 1 diabetes to lower A1c, body weight and insulin doses, when added to insulin.
It is important to recognize individual autonomy of people living with T1D and although this recommendation is not a strong treatment recommendation, as the authors suggest, it is a conditional recommendation that allows for people with T1D to consider their individual health goals and to advocate for treatments that work best for them:
In adults, adjunctive therapy, such as metformin, glucagon-like peptide-1 receptor agonists (GLP1-RA) or sodium-glucose cotransporter-2 inhibitors (SGLT2i), may be considered in addition to insulin to meet individual treatment goals while employing strategies to support safety, efficacy, and tolerability of these medications.
Breakthrough T1D Canada is pleased to see these updated clinical guidelines that will empower people living with T1D to have important conversations with their healthcare provider(s) to discuss the best options for their care. We will continue to advocate for coverage of these therapies and devices so that everyone has access to the treatments and devices that meet their individual health needs.
Patient choice is paramount for diabetes devices, treatments, insulins and insulin administration, as such, Breakthrough T1D will continue to advocate and work with the T1D community to ensure better access, affordability and choice for all Canadians living with T1D.
If you would like to learn more about the updated guidelines, automated insulin delivery devices or adjunctive therapies, please speak to your diabetes health care provider.
Image reproduced in its entirety from Halperin, Wicklow, Amed, et al., on behalf of the Diabetes Canada Clinical Practice Guidelines Steering Committee (2025) Canadian Journal of Diabetes; 49, 5-18. https://www.canadianjournalofdiabetes.com/article/S1499-2671(25)00001-2/fulltext



