Breakthrough T1D Canada is thrilled to continue a successful partnership with the Canadian Islet Research and Training Network (CIRTN) and announce a third cohort of co-funded trainees beginning in 2025.
CIRTN was established in 2020 as a world-class training and research network with joint contributions from the University of Alberta, University of British Columbia, University of Manitoba, Université de Montréal, Institut de Recherches Cliniques de Montréal, and the University of Toronto and now includes 12 institutions from across Canada. Breakthrough T1D Canada has partnered with CIRTN to leverage funding to this network from the National Science and Engineering Research Council – Collaborative Research and Training Experience (NSERC-CREATE) program.
See 2023 cohort trainees here. See 2024 cohort trainees here.
Dr. Summer Helmi
Postdoctoral Fellow
Supervisor: Dr. Andrew Pepper, University of Alberta
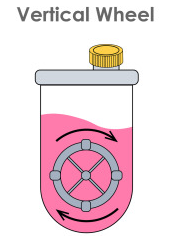
Optimizing Neonatal Porcine Islet Differentiation Using the PBS Mini Vertical-Wheel ® Bioreactor: Advancing Xenotransplantation for Type 1 Diabetes
This innovative project explores a promising approach to treating type 1 diabetes using neonatal porcine islets (NPIs) as a cell replacement therapy. While NPIs offer advantages over other methods, optimizing their production for clinical use remains a challenge. The research compares NPI culturing in conventional static suspension methods (i.e., growing the stem cells in a suspension medium in a petri dish or beaker) with dynamic vertical wheel bioreactors (i.e., growing the same cells in a device that controls movement to mimic the body’s cell growth conditions). Early results suggest that dynamic culture systems may significantly enhance NPI production and quality, potentially improving islet morphology, viability, and functionality. This work could overcome critical hurdles in NPI production, bringing us closer to a more effective and accessible treatment for type 1 diabetes. The project aims to advance porcine islet differentiation strategies and transform diabetes care worldwide.
IMAGE: Suspension culture. Vertical wheel bioreactor.
Dr. Nayara Rampazzo Morelli
Postdoctoral Fellow
Supervisor: Dr. Peter Thompson, University of Manitoba
Investigating a potential drug target in human beta cells during type 1 diabetes
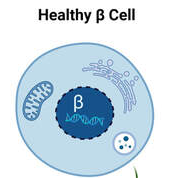
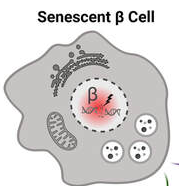
Type 1 diabetes (T1D) is well known for its autoimmune aspects which leads to loss of most of the beta cells in the body and insulin deficiency. How the process of beta cell loss occurs remains uncertain, but recent work indicates that the accumulation of stressed beta cells can accelerate T1D onset. Relieving the stressed beta cells slows the development of T1D in animal models but targets are lacking in humans, preventing us from determining whether this treatment approach could be moved to the clinic for T1D patients. The aim of this study is to evaluate a particular drug target in stressed human beta cells to determine whether this approach could be used to delay the progression of T1D or improve symptoms in people living with T1D.
(image from Dr. Thompson)
IMAGE: health beta cell. Senescent (dying) beta cell.
Dr. Shreyasi Sarkar
Postdoctoral Fellow,
Supervisor: Dr. Gareth Lim, Université de Montréal
Assessing the potential of targeting 14-3-3z to restore functional beta cell mass
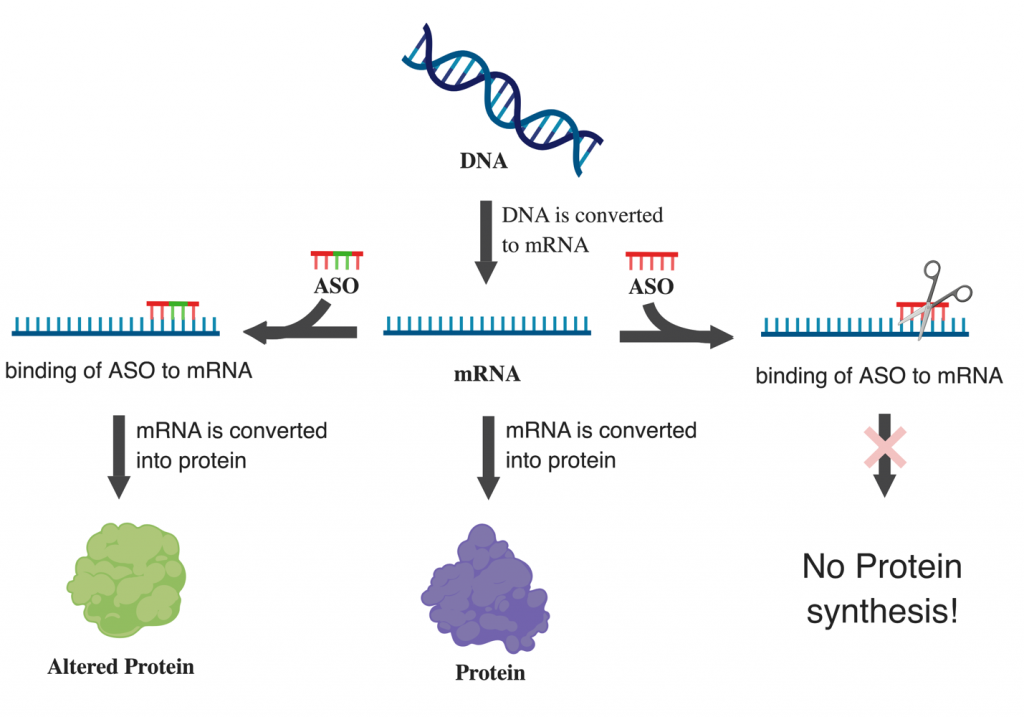
Dr. Sarkar’s research focuses on identifying new ways to increase beta cell number (mass) to treat T1D. I study a protein called 14-3-3z, and earlier work from Dr. Lim showed that targeting this protein during early development in mice improved insulin secretion and beta cell mass, making this protein a promising target for diabetes treatment. Dr. Sarkar is now exploring a new approach using Antisense Oligonucleotides (a synthetic strand of nucleotide that can modulate protein expression) to target this protein after birth and explore whether similar improvements can be achieved. This may represent a new approach to treat diabetes.
(Image from https://www.ataxia.org/)